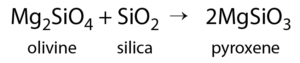Chapter 7 Igneous Rocks
Adapted from Physical Geology, First University of Saskatchewan Edition (Karla Panchuk) and Physical Geology (Steven Earle)
. Click the image for more attributions._](figures/07-igneous-rocks/figure-7-1.png)
Figure 7.1: Lava lake of Mount Nyiragongo, a volcano in the Democratic Republic of Congo. Igneous rocks form when melted rock freezes. Source: Karla Panchuk (2018) CC BY-NC-SA 4.0. Photo by Baron Reznik (2015) CC BY-NC-SA 2.0 view source. Click the image for more attributions.
Learning Objectives
After reading this chapter and answering the review questions at the end, you should be able to:
- Explain partial melting and the geological processes that lead to melting.
- Describe the range of chemical compositions of magmas.
- Discuss the processes that take place during magma cooling, and the order of crystallization in Bowen’s reaction series.
- Explain how fractional crystallization and partial melting alter magma composition.
- Classify igneous rocks according to the proportions of minerals within them.
- Describe the origins of aphanitic, phaneritic, and porphyritic textures
- Classify plutons according to their shapes and relationships to surrounding rocks.
- Explain how chilled margins form.
7.1 Magma and How It Forms
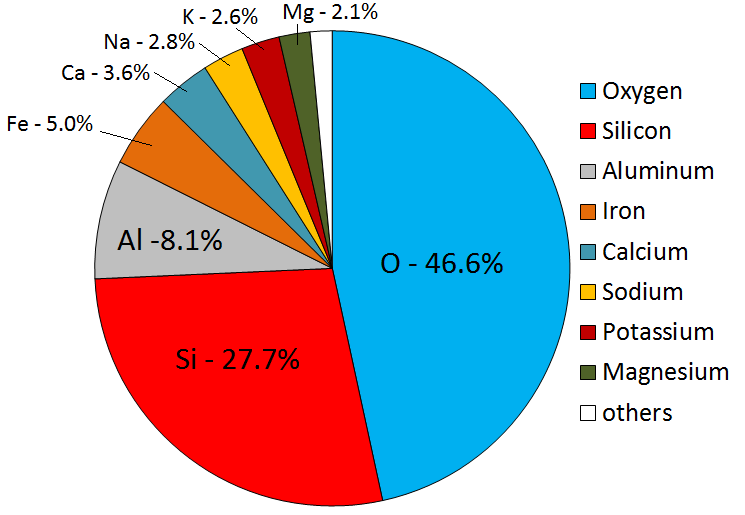
Igneous rocks form when melted rock cools. Melted rock originates within Earth as magma. Magma compositions vary, but will have eight main elements in different proportions. The most abundant elements are oxygen and silicon, followed by aluminum, iron, calcium, sodium, magnesium, and potassium. These eight elements are also the most abundant in Earth’s crust (Figure 7.2). All magmas have varying proportions of lighter elements such as hydrogen, carbon, and sulphur. Lighter elements are converted into gases like water vapour, carbon dioxide, hydrogen sulphide, and sulphur dioxide as the magma cools.
_](figures/07-igneous-rocks/figure-7-2.png)
Figure 7.2: Average composition of Earth’s crust by mass. Source: Steven Earle (2016) CC BY 4.0 view source
Magma composition depends on the composition of the rocks that melted to form the magma, and on the conditions under which the melting happened. Most igneous rock in Earth’s crust comes from magmas that formed through partial melting of existing rock, either in the upper mantle or the crust. During partial melting, only some of the minerals within a rock melt. This happens because different minerals have different melting temperatures. The melt is less dense than the surrounding rock, and will percolate upward without the source rock having melted completely. The result is magma with a different composition than the original rock. Partial melting produces melt that has more silica than the original rock, because minerals higher in silica have lower melting points.
To see how partial melting works, consider the mix of materials in Figure 7.3a. It contains white blocks of candle wax, black plastic pipe, green beach glass, and pieces of aluminum wire. When the mixture is heated to 50 °C in a warm oven, the wax melts into a clear liquid (Figure 7.3b), but the other materials remain solid. This is partial melting.
_](figures/07-igneous-rocks/figure-7-3.png)
Figure 7.3: An experiment to illustrate partial melting. (a) The original components are white candle wax, black plastic pipe, green beach glass, and aluminum wire. (b) After heating to 50˚C for 30 minutes only the wax has melted. (c) After heating to 120˚C for 60 minutes much of the plastic has melted and the two liquids have mixed. (d) The liquid has been poured off and allowed to cool, making a solid with a different overall composition from the original mixture. Source: Steven Earle (2015) CC BY 4.0 view source
When the mixture is heated to 120 °C, the plastic melts and mixes with the wax, but the aluminum and glass still remain solid (Figure 7.3c). This is still considered partial melting because solid materials remain. When the plastic and wax mixture is poured into a separate container and allowed to cool, the resulting solid has a very different composition from the original mixture (Figure 7.3d). The plastic and wax are analogous to more silica-rich minerals with relatively lower melting points than other minerals in the same rock.
Of course, partial melting in the real world isn’t as simple as the example in Figure 7.3. Many rocks are much more complex than the four-component system used here. Some mineral components of rocks may have similar melting temperatures, and begin to melt at the same time. The melting temperature of a mineral may change in the presence of other minerals. Also, when rocks melt, the process can take millions of years, unlike the 90 minutes required to melt the pipe and wax in the experiment in Figure 7.3.
7.1.1 Why Rocks Melt
The magma that is produced by partial melting is less dense than the surrounding rock. Magma from partial melting of mantle rocks rises upward through the mantle, and may pool at the base of the crust, or rise through the crust. Moving magma carries heat with it, and some of that heat is transferred to surrounding rocks. If the melting temperature of a rock is less than the temperature of the magma, the rock will begin to melt, and the composition of the magma may change to reflect a mixture of sources. But adding heat is not the only way to trigger melting.
7.1.1.1 Decompression Melting
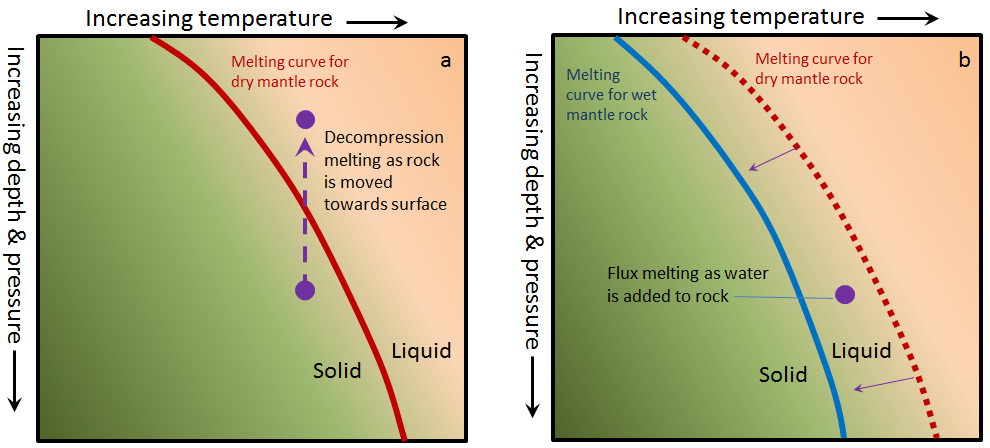
Earth’s mantle is almost entirely solid rock, in spite of temperatures that would cause rock at Earth’s surface to melt. Mantle rock remains solid at those temperatures because the rock is under high pressure. This means that melting can be triggered without adding heat if the rock is already hot enough, and the pressure is reduced (Figure 7.4, left, white dashed boxes). Melting triggered by a reduction in pressure is called decompression melting.
_](figures/07-igneous-rocks/figure-7-4.png)
Figure 7.4: Melting triggers. Left- Decompression melting occurs when rock rises or the overlying crust thins. Right- Flux-induced melting occurs when volatile compounds such as water are added. Source: Karla Panchuk (2018) CC BY 4.0. Modified after Steven Earle (2016) CC BY 4.0 view source
Pressure is reduced when mantle rocks move upward due to convection, or rise as a plume within the mantle. Pressure is also reduced where the crust thins, such as along rift zones.
7.1.1.2 Flux-induced Melting
When a substance such as water is added to hot rocks, the melting points of the minerals within those rocks decreases. If a rock is already close to its melting point, the effect of adding water can be enough to trigger partial melting. The added water is a flux, and this type of melting is called flux-induced melting. In Figure 7.4 (right), the rock (represented by the dashed box) is not hot enough to be right of the line where dry mantle rocks melt, but it is to the right of the line where wet mantle rocks melt.
Flux-induced partial melting of rock happens in subduction zones. Minerals are transformed by chemical reactions under high pressures and temperatures, and a by-product of those transformations is water. Relatively little water is required to trigger partial melting. In laboratory studies of the conditions of partial melting in the Japanese volcanic arc, rocks with only 0.2% of their weight consisting of water melted by up to 25%.
7.1.2 Cooling Magma Becomes More Viscous
Viscosity refers to the ease with which a substance flows. A substance with low viscosity is runnier than a substance with high viscosity. At temperatures over 1300°C, most magma is entirely liquid because there is too much energy for the atoms to bond together. As magma loses heat to the surrounding rocks and its temperature drops, things start to change. Silicon and oxygen combine to form silica tetrahedra. With further cooling, the tetrahedra start to link together into chains, or polymerize. These silica chains make the magma more viscous. Magma viscosity has important implications for the characteristics of volcanic eruptions.
Exercise: Making Magma Viscous
This is a quick and easy experiment that you can do at home to help you understand the properties of magma. It will only take about 15 minutes, and all you need is half a cup of water and a few tablespoons of flour.
If you’ve ever made gravy, white sauce, or roux, you’ll know how this works.
Place 1/2 cup (125 mL) of water in a saucepan over medium heat. Add 2 teaspoons (10 mL) of white flour and stir while continuing to heat the mixture until boiling. The white flour represents silica. The mixture should thicken like gravy because the gluten in the flour becomes polymerized into chains during this process.
Now add more “silica” to see how this changes the viscosity of your magma: take another 4 teaspoons (20 mL) of flour and mix it thoroughly with 4 teaspoons (20 mL) of water in a cup. Add that mixture to the rest of the water and flour in the saucepan. Stir while bringing it back up to nearly boiling temperature, and then allow it to cool. This mixture should slowly become much thicker (Figure 7.5) because there is more gluten, and more chains have formed.
_](figures/07-igneous-rocks/figure-7-5.jpg)
Figure 7.5: Thick mixture of flour and water. Source: Steven Earle (2016) CC BY 4.0 view source
7.2 Crystallization of Magma
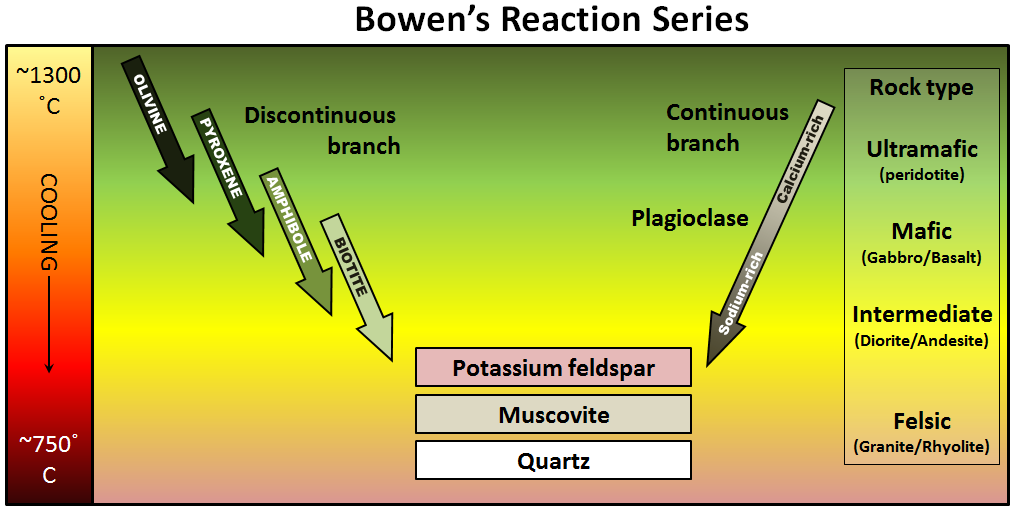
The minerals that make up igneous rocks crystallize (solidify, freeze) at a range of different temperatures. This explains why cooling magma can have some crystals within it and yet remain predominantly liquid. The sequence in which minerals crystallize from a magma as it cools is known as Bowen’s reaction series (Figure 7.6).
_](figures/07-igneous-rocks/figure-7-6.png)
Figure 7.6: Bowen’s reaction series describe the sequence in which minerals form as magma cools. Source: Steven Earle (2016) CC BY 4.0 view source
7.2.1 How Did We Get Bowen’s Reaction Series?
Understanding how the reaction series was derived is key to understanding what it means.

Figure 7.7: Norman Bowen in his laboratory. Source: University of Chicago Photographic Archive, apf1-00841, Special Collections Research Center, University of Chicago Library. Click the image for source and terms of use.
Norman Levi Bowen (Figure 7.7) was born in Kingston Ontario. He studied geology at Queen’s University and then at Massachusetts Institute of Technology. In 1912 he joined the Carnegie Institution in Washington, D.C., where he carried out ground-breaking experiments into how magma cools.
Working mostly with mafic magmas (magmas rich in iron and magnesium), he determined the order of crystallization of minerals as the temperature drops. First, he melted the rock completely in a specially made kiln. Then he allowed it to cool slowly to a specific temperature before quenching (cooling it quickly) so that no new minerals could form. The rocks that formed were studied under the microscope and analyzed chemically. This was done over and over, each time allowing the magma to cool to a lower temperature before quenching.
The result of these experiments was the reaction series which, even a century later, is still an important basis for our understanding of igneous rocks.
7.2.2 Discontinuous and Continuous Series
Bowen’s reaction series (Figure 7.6) has two pathways for minerals to form as magma cools: on the left is the discontinuous series. This refers to the fact that one mineral is transformed into a different mineral through chemical reactions. On the right is the continuous series, where plagioclase feldspar goes from being rich in calcium to being rich in sodium.
7.2.2.1 Continuous Series
At about the point where pyroxene begins to crystallize, plagioclase feldspar also begins to crystallize. At that temperature, the plagioclase is calcium-rich (toward the anorthite end-member). As the temperature drops, and providing that there is sodium left in the magma, the plagioclase that forms is a more sodium-rich variety (toward the albite end-member). The series is continuous because the mineral is always plagioclase feldspar, but the series involves a transition from calcium-rich to sodium-rich.
When cooling happens relatively quickly, instead of getting crystals which are of uniform composition, individual plagioclase crystals can be zoned from calcium-rich in the centre to more sodium-rich around the outside (Figure 7.8). This occurs because calcium-rich early-forming plagioclase crystals become coated with progressively more sodium-rich plagioclase as the magma cools.
/ [view context](https://open.agh.edu.pl/mod/page/view.php?id=914)_](figures/07-igneous-rocks/figure-7-8.jpeg)
Figure 7.8: Plagioclase crystal exhibiting compositional zones. Source: Akademia Górniczo-Hutnicza w Krakowie Otwartych Zasobów Edukacyjnych (n.d.) CC BY-NC-SA view source/ view context
7.2.2.2 Discontinuous Series
Olivine begins to form at just below 1300°C, but as the temperature drops, olivine becomes unstable. The early-forming olivine crystals react with silica in the remaining liquid and are converted into pyroxene, something like this:
As long as there is silica remaining and the rate of cooling is slow, this process continues down the discontinuous branch: olivine reacts to form pyroxene, and the pyroxene reacts to form amphibole. Under the right conditions amphibole will form to biotite. Finally, if the magma is quite silica-rich to begin with, there will still be some left at around 750 °C to 800 °C, and from this last magma, potassium feldspar, quartz, and maybe muscovite mica will form.
Notice that the sequence of minerals that form goes from isolated tetrahedra (olivine) toward increasingly complex arrangements of silica tetrahedra. Pyroxene consists of single chains, amphibole has double chains, mica has sheets of tetrahedra, and potassium feldspar and quartz at the bottom of the series have tetrahedra connected to each other in three dimensions.
If the magma cools enough, the first minerals to form will be completely used up in later chemical reactions. This is why igneous rocks do not normally have both olivine (at the top of the series) and quartz (at the bottom). Exceptions can occur when rocks that crystallized early in the series come into contact with magmas representing compositions later in the series, such as with the dark green olivine-rich xenoliths included within the quartz- and feldspar-rich rock in Figure 7.9 The dark line around the xenoliths is amphibole, which formed as the olivine reacted with the melt. In some of the smaller xenoliths within this boulder, the olivine has been completely transformed into amphibole.
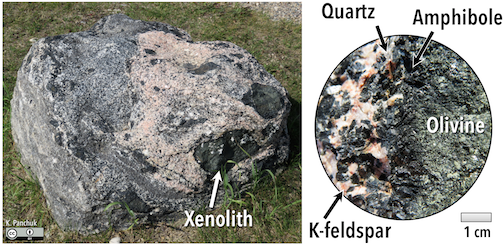
Figure 7.9: Boulder with olivine-rich xenoliths surrounded by silica-rich rock. Black rims on the xenoliths are where the olivine has reacted with the silica-rich melt, forming amphibole. Right- Enlarged view of the amphibole reaction rim. Source: Karla Panchuk (2018) CC BY 4.0
7.2.3 Magma Composition: Mafic, Intermediate, and Felsic
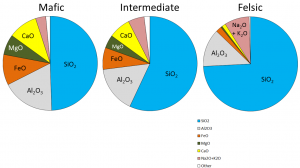
The composition of the original magma determines how far the reaction process can continue before all of the magma is used up. In other words, it determines which minerals will form. Magma compositions are reported in terms of the fraction of mass of oxides (e.g., Al2O3 rather than just Al; Figure 7.10). On average, mafic9 magma (Figure 7.10, left) is approximately half SiO2 by mass, and more than 25% iron, magnesium, and calcium oxides by mass. Average felsic10 magmas (Figure 7.10, right) are closer to 75% SiO2 by mass, and have approximately 5% iron, magnesium, and calcium oxides. Sodium and potassium oxides account for approximately 10% of felsic magmas by mass, but only 5% of mafic magmas. Magmas that fall between mafic and felsic magmas have an __intermediate __composition (Figure 7.10, centre).
_](figures/07-igneous-rocks/figure-7-10.png)
Figure 7.10: Chemical compositions of typical mafic, intermediate, and felsic magmas. Source: Karla Panchuk (2018) CC BY 4.0 modified after Steven Earle (2016) CC BY 4.0 view source
Exercise: Mafic, Intermediate, or Felsic?
The proportions of the main chemical components of felsic, intermediate, and mafic magmas are listed in the table below. (The values are similar to those shown in Figure 7.10).
| Oxide | Felsic Magma | Intermediate Magma | Mafic Magma |
|---|---|---|---|
| SiO2 | 65-75% | 55-65% | 45-55% |
| Al2O3 | 12-16% | 14-18% | 14-18% |
| FeO | 2-4% | 4-8% | 8-12% |
| CaO | 1-4% | 4-7% | 7-11% |
| MgO | 0-3% | 2-6% | 5-9% |
| Na2O | 2-6% | 3-7% | 1-3% |
| K2O | 3-5% | 2-4% | 0.5-3% |
Chemical composition by mass for four rock samples are shown in the following table. Compare these with those in the table above to determine whether each of these samples is felsic, intermediate, or mafic.
| Sample | SiO2 | Al2O3 | FeO | CaO | MgO | Na2O | K2O |
|---|---|---|---|---|---|---|---|
| 1 | 55% | 17% | 5% | 6% | 3% | 4% | 3% |
| 2 | 74% | 14% | 3% | 3% | 0.5% | 5% | 4% |
| 3 | 47% | 14% | 8% | 10% | 8% | 1% | 2% |
| 4 | 65% | 14% | 4% | 5% | 4% | 3% | 3% |
7.2.4 What Determines the Composition of Magma?
7.2.4.1 Why Is There No Ultramafic Magma Anymore?
Refer back to Bowen’s reaction series in Figure 7.6. Notice that on the far right-hand side of the diagram under “Rock Types,” mafic, intermediate, and felsic magma compositions are listed. At the very top of the list is ultramafic. Ultramafic rocks have higher MgO than mafic rocks, and even less SiO2.
The vast majority of silicate rocks in Earth’s lithosphere are ultramafic rocks, because the mantle is composed of ultramafic rock. However, ultramafic magma is not encountered in modern volcanic environments, and ultramafic rocks are relatively rare at Earth’s surface. The reason is that although Earth was once hot enough to have ultramafic magma, it is no longer hot enough to melt ultramafic rocks. Ultramafic volcanic rocks—called komatiites—do exist, but with two notable exceptions, the youngest of these is 2 billion years old.11
7.2.4.2 Partial Melting Makes Magma That Is Richer in Silica
In partial melting, some components of a mixture melt before others do. In the case of mafic magma, it is produced when ultramafic rocks undergo partial melting. In general, silicate minerals with more silica will melt before those with less silica. This means the partial melt will have more silica than the rock as a whole.
7.2.4.3 Fractional Crystallization Also Makes Magma Richer In Silica
A number of processes that take place within a magma chamber can affect the types of rocks that form once magma cools and crystallizes. If the magma has a low viscosity— which is likely if the magma is mafic—the crystals that form early, such as olivine (Figure 7.11a), may slowly settle toward the bottom of the magma chamber (Figure 7.11b). This process is called fractional crystallization.
_](figures/07-igneous-rocks/figure-7-11.png)
Figure 7.11: Formation of a zoned magma chamber. a- Olivine crystals form. b- Olivine crystals settle to the base of the magma chamber, leaving the upper part of the chamber richer in silica. c- Olivine crystals remelt, making magma at the base of the chamber more mafic. Source: Steven Earle (2015) CC BY 4.0 view source
The formation of olivine removes iron- and magnesium-rich components, leaving the overall composition of the magma near the top of the magma chamber more felsic. The crystals that settle might either form an olivine-rich layer near the bottom of the magma chamber. Or, because the lower part of the magma chamber is likely to be hotter than the upper part, the crystals might remelt. If remelting happens, crystal settling will make the magma at the bottom of the chamber more mafic than it was to begin with (Figure 7.11c).
7.2.4.4 Magma Composition Also Changes When Other Rocks Are Melted And Mixed In
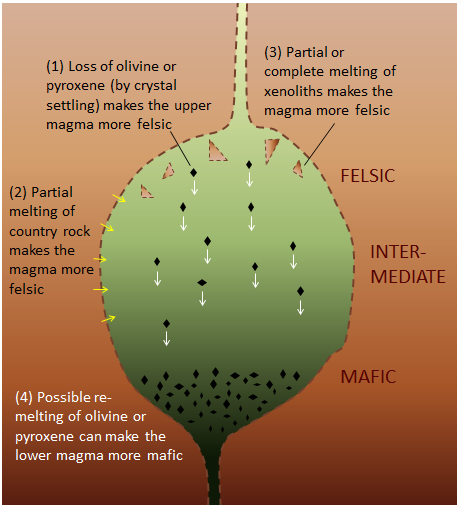
Magma chambers aren’t isolated from their surroundings. If the rock in which the magma chamber is located (called the country rock) is more felsic than the magma, the country rock may also melt, adding to the magma already in the magma chamber (Figure 7.12). Sometimes magma carries fragments of unmelted rock, called xenoliths, within it. Melting of xenoliths can also alter the composition of magma, as can re-melting of crystals that have settled out of the magma.
_](figures/07-igneous-rocks/figure-7-12.png)
Figure 7.12: The composition of magma in a magma chamber is affected by fractional crystallization within the magma chamber, but it can also be affected by partial melting of the rock surrounding the magma chamber, melting of xenoliths within the magma, or re-melting of crystals that have settled to the bottom of the magma chamber. Source: Steven Earle (2015) CC BY 4.0 view source
7.3 Classification of Igneous Rocks
7.3.1 Classification By Mineral Abundance
Igneous rocks can be divided into four categories based on their chemical composition: felsic, intermediate, mafic, and ultramafic. The diagram of Bowen’s reaction series (Figure 7.6) shows that differences in chemical composition correspond to differences in the types of minerals within an igneous rock. Igneous rocks are given names based on the proportion of different minerals they contain. Figure 7.13 is a diagram with the minerals from Bowen’s reaction series, and is used to decide which name to give an igneous rock.
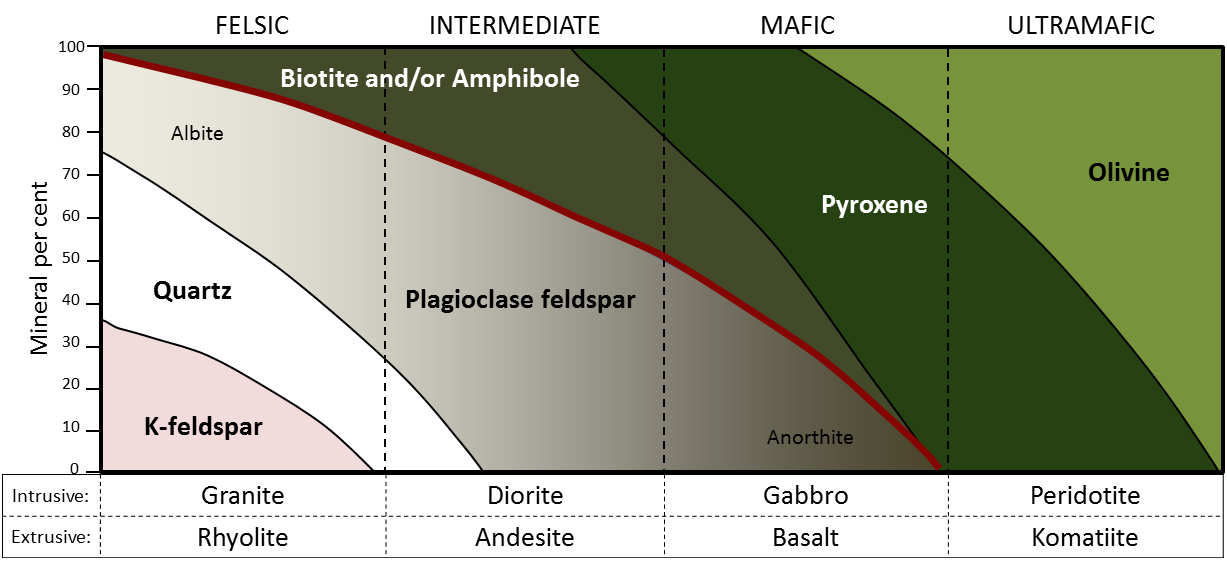 and others, with photos by R. Weller/Cochise College. Click the image for links to photos and notes on image construction. [High-resolution version](https://openpress.usask.ca/app/uploads/sites/29/2018/06/igneous-rock-classification_revised.png)._](figures/07-igneous-rocks/figure-7-13.png)
Figure 7.13: Classification diagram for igneous rocks. Igneous rocks are classified according to the relative abundances of minerals they contain. A given rock is represented by a vertical line in the diagram. In the mafic field, the arrows represent a rock containing 48% pyroxene and 52% plagioclase feldspar. The name an igneous rock gets depends not only on composition, but on whether it is intrusive or extrusive. Source: Karla Panchuk (2018) CC BY-NC-SA 4.0, modified after Steven Earle (2015) CC BY 4.0 view source and others, with photos by R. Weller/Cochise College. Click the image for links to photos and notes on image construction. High-resolution version.
To see how Figure 7.13 works, first notice the scale in percent along the vertical axis. The interval between each tick mark represents 10% of the minerals within a rock by volume. An igneous rock can be represented as a vertical line drawn through the diagram, and the vertical scale used to break down the proportion of each mineral it contains. For example, the arrows in the mafic field of the diagram represent a rock containing 48% pyroxene and 52% plagioclase feldspar. An igneous rock at the boundary between the mafic and ultramafic fields (marked with a vertical dashed line) would have approximately 20% olivine, 50% pyroxene, and 30% Ca-rich plagioclase feldspar by volume.
7.3.2 Classification By Grain Size
The name an igneous rock gets also depends on whether it cools within Earth (an intrusive or plutonic igneous rock), or whether it cools on the Earth’s surface after erupting from a volcano (an extrusive or volcanic igneous rock). For example, a felsic intrusive rock is called granite, whereas a felsic extrusive rock is called rhyolite. Granite and rhyolite have the same mineral composition, but their grain size gives each a distinct appearance.
The key difference between intrusive and extrusive igneous rocks—the size of crystals making them up—is related to how rapidly melted rock cools. The longer melted rock has to cool, the larger the crystals within it can become. Magma cools much slower within Earth than on Earth’s surface because magma within Earth is insulated by surrounding rock. Notice that in Figure 7.13, the intrusive rocks have crystals large enough that you can see individual crystals—either by identifying their boundaries, or seeing light reflecting from a crystal face. A rock with individual crystals that are visible to the unaided eye has a phaneritic or coarse-grained texture. The extrusive rocks in the second row have much smaller crystals. The crystals are so small that individual crystals cannot be distinguished, and the rock looks like a dull mass. A rock with crystals that are too small to see with the unaided eye has an aphanitic or fine-grained texture. Table 7.1 summarizes the key differences between intrusive and extrusive igneous rocks.
| Parameter | Magma | Lava |
|---|---|---|
| Cooling location | cools within Earth | cools on Earth’s surface |
| Terminology | Intrusive/ plutonic | Extrusive/ volcanic |
| Cooling rate | Slow: surrounding rocks insulate the magma chamber. | Rapid: heat is exchanged with the atmosphere. |
| Texture | Phaneritic (coarse-grained): individual crystals are large enough to see without magnification. | Aphanitic (fine-grained): crystals are too small to see without magnification. |
What this means is that two igneous rocks comprised of exactly the same minerals, and in the exactly the same proportions, can have different names. A rock of intermediate composition is diorite if it is course-grained, and andesite if it is fine-grained. A mafic rock is gabbro if it is course-grained, and basalt if fine-grained. The course-grained version of an ultramafic rock is peridotite, and the fine-grained version is komatiite. It makes sense to use different names because rocks of different grain sizes form in different ways and in different geological settings.
7.3.2.1 Does This Mean an Igneous Rock Can Only Have One Grain Size?
No. Something interesting happens when there is a change in the rate at which melted rock is cooling. If magma is cooling in a magma chamber, some minerals will begin to crystallize before others do. If cooling is slow enough, those crystals can become quite large.
Now imagine the magma is suddenly heaved out of the magma chamber and erupted from a volcano. The larger crystals will flow out with the lava. The lava will then cool rapidly, and the larger crystals will be surrounded by much smaller ones. An igneous rock with crystals of distinctly different size (Figure 7.14) is said to have a porphyritic texture, or might be referred to as a porphyry. The larger crystals are called phenocrysts, and the smaller ones are referred to as the groundmass.
_](figures/07-igneous-rocks/figure-7-14.png)
Figure 7.14: Porphyritic rhyolite with quartz and potassium feldspar phenocrysts within a dark groundmass. Porphyritic texture (when different crystal sizes are present) is an indication that melted rock did not cool at a constant rate. Source: Karla Panchuk (2018) CC BY-NC-SA 4.0. Photo by R. Weller/Cochise College (2011) view source
Exercise: Which Mineral Will the Phenocryst Be?
As a magma cools below 1300°C, minerals start to crystallize within it. If the magma is then erupted, the rest of the liquid will cool quickly to form a porphyritic texture. The rock will have some relatively large crystals (phenocrysts) of the minerals that crystallized early, and the rest will be very fine-grained or even glassy. Using the diagram shown here, predict what phenocrysts might be present where the magma cooled as far as line a. Which would be present where magma cooled to line b?
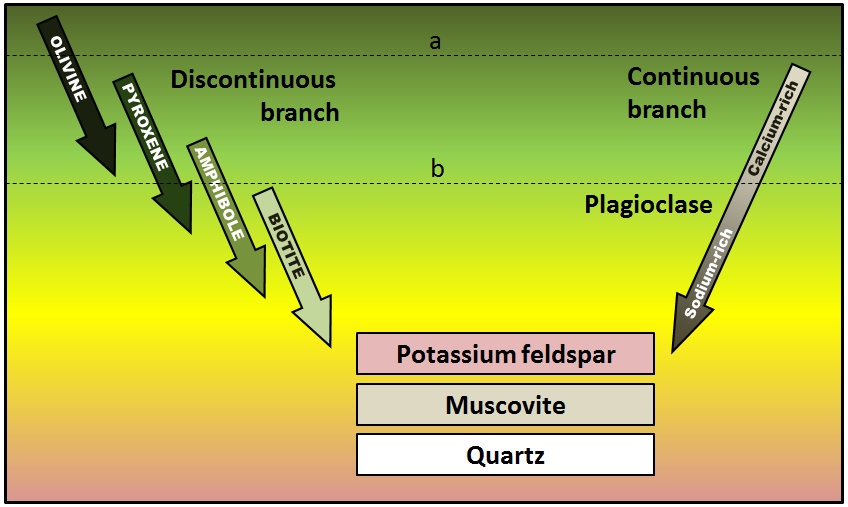_](figures/07-igneous-rocks/figure-7-15.png)
Figure 7.15: Bowen’s reaction series. Source: Steven Earle (2015) CC BY 4.0 view source
7.3.3 Classifying Igneous Rocks According to the Proportion of Dark Minerals
If you unsure of which minerals are present in an intrusive igneous rock, there is a quick way to approximate the composition of that rock. In general, igneous rocks have an increasing proportion of dark minerals as they become more mafic (Figure 7.16).
_](figures/07-igneous-rocks/figure-7-16.png)
Figure 7.16: Simplified igneous rock classification according to the proportion of light and dark (or ferromagnesian) minerals. Source: Karla Panchuk (2018) CC BY 4.0, modified after Steven Earle (2015) CC BY 4.0 view source
The dark-coloured minerals are those higher in iron and magnesium (e.g., olivine, pyroxene, amphibole, biotite), and for that reason they are sometimes referred to collectively as ferromagnesian minerals. By estimating the proportion of light minerals to dark minerals in a sample, it is possible to place that sample in Figure 7.16. Graphical scales are used to help visualize the proportions of light and dark minerals (Figure 7.17).
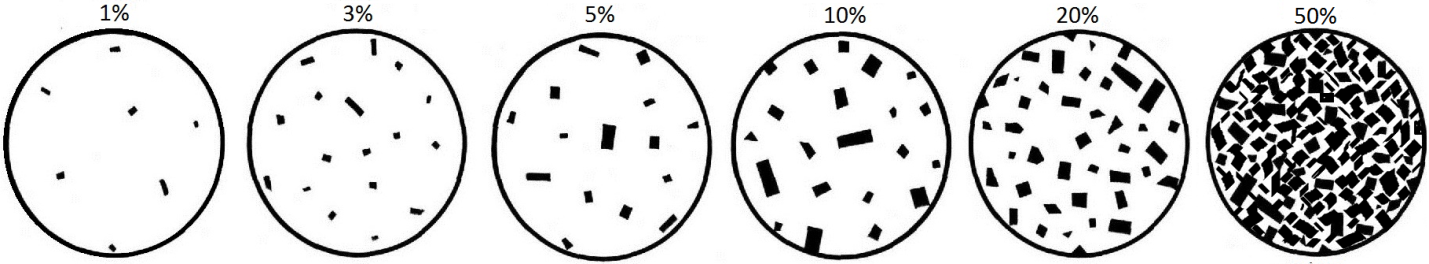_](figures/07-igneous-rocks/figure-7-17.png)
Figure 7.17: A guide for estimating the proportion of dark minerals in an igneous rock. Source: Karla Panchuk (2018) CC BY 4.0, modified after Steven Earle (2015) view source
It is important to note that estimating the proportion of dark minerals is only approximate as a means for identifying igneous rocks. One problem is that plagioclase feldspar is light-coloured when it is sodium-rich, but can appear darker if it is calcium-rich. Plagioclase feldspar is not ferromagnesian, so it falls in the non-ferromagnesian (light minerals) region in Figure 7.16 even when it has a darker colour.
Exercise: Classifying Igneous Rocks by the Proportion of Dark Minerals
The four igneous rocks shown below have differing proportions of ferromagnesian silicates (dark minerals). Estimate the proportion of dark minerals using the guide in Figure 7.17, and then use Figure 7.16 to determine the likely rock name for each one.
_](figures/07-igneous-rocks/figure-7-18.png)
Figure 7.18: Identify these rocks by estimating the proportion of dark minerals in each sample. Source: Karla Panchuk (2018) CC BY 4.0, modified after Steven Earle (2015) CC BY 4.0 view source
7.3.4 Classifying Igneous Rocks When Individual Crystals Are Not Visible
The method of estimating the percentage of minerals works well for phaneritic igneous rocks, in which individual crystals are visible with little to no magnification. If an igneous rock is porphyritic but otherwise aphanitic (e.g., Figure 7.14), the minerals present as phenocrysts give clues to the identity of the rock. However, there are cases where mineral composition cannot be determined by looking at visible crystals. These include volcanic rocks without phenocrysts, and glassy igneous rocks.
7.3.4.1 Volcanic Rocks Without Phenocrysts
In the absence of visible crystals or phenocrysts, volcanic rocks are be classified on the basis of colour and other textural features. As you may have noticed in Figure 7.13, the colour of volcanic rocks goes from light to dark as the composition goes from felsic to mafic. Rhyolite is often a tan or pinkish colour, andesite is often grey, and basalt ranges from brown to dark green to black (Figure 7.19).
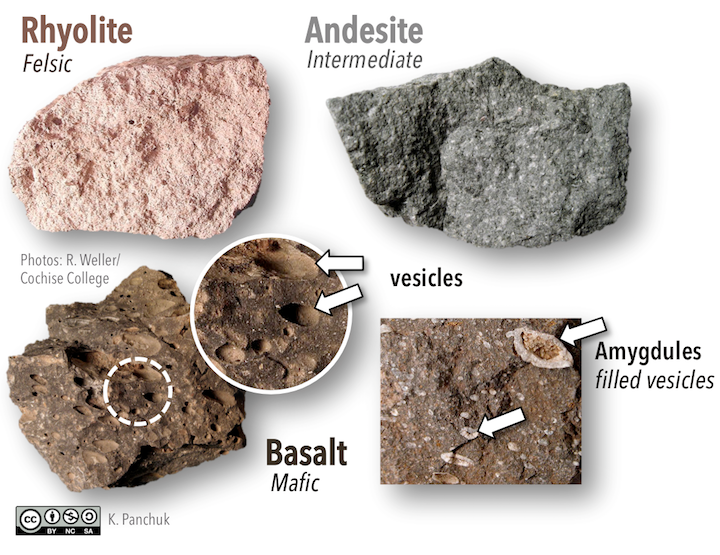
Figure 7.19: In volcanic igneous rocks, individual crystals are not visible. Colours change from light to dark as the composition of the rocks go from felsic to mafic. Vesicles and amygdules are common characteristics of basalt. Source: Karla Panchuk (2018) CC BY-NC-SA 4.0. Photos by R. Weller/ Cochise College. Click the image for links to photos.
Basalt often shows textural features related to lava freezing around gas bubbles. When magma is underground, pressure keeps gases dissolved, but once magma has erupted, the pressure is much lower. Gases dissolved in the lava are released, and bubbles can develop. When lava freezes around the bubbles, vesicles are formed (circular inset in 7.19). If the vesicles are later filled by other minerals, the filled vesicles are called amygdules (box inset in Figure 7.19).
7.3.4.2 Glassy Volcanic Rocks
Crystal size is a function of cooling rate. The faster magma or lava cools, the smaller the crystals it contains. It is possible for lava to cool so rapidly that no crystals can form. The result is called volcanic glass. Volcanic glass can be smooth like obsidian or vesicular like scoria (mafic) and pumice (felsic; Figure 7.20). Pumice can float on water because of its low-density felsic composition and enclosed vesicles.

Figure 7.20: Glassy volcanic rocks. Obsidian has a glassy lustre, but scoria and pumice are highly vesicular. Source: Karla Panchuk (2018) CC BY-NC-SA 4.0. Photos by R. Weller/Cochise College. Click the image for links to photos.
7.4 Intrusive Igneous Rocks
In most cases, a body of hot magma is less dense than the rock surrounding it, so it has a tendency to creep upward toward the surface. It does so in a few different ways:
- Filling and widening existing cracks
- Melting the surrounding rock
- Pushing the rock aside (where the rock is hot enough and under enough pressure to deform without breaking)
- Breaking the rock
When magma forces itself into cracks, breaks off pieces of rock, and then envelops them, this is called stoping. The resulting fragments are xenoliths12. Xenoliths may appear as dark patches within a rock (Figure 7.21).
](figures/07-igneous-rocks/figure-7-21.jpg)
Figure 7.21: Xenoliths of mafic rock in granite, Victoria, B.C. The fragments of dark rock have been broken off and incorporated into the light-coloured granite._ Source: Steven Earle (2015) CC BY 4.0_ view source
Some of the magma may reach the surface, resulting in volcanic eruptions, but most cools within the crust. The resulting body of rock is called a pluton.13 Plutons can have different shapes and different relationships with the surrounding country rock (Figure 7.22). These characteristics determine what name the pluton is given.
Large, irregularly shaped plutons are called stocks or batholiths, depending upon their size. Tabular plutons are called dikes if they cut across existing structures, and sills if they are parallel to existing structures. Laccoliths are like sills, except they have caused the overlying rocks to bulge upward. Pipes are cylindrical conduits.
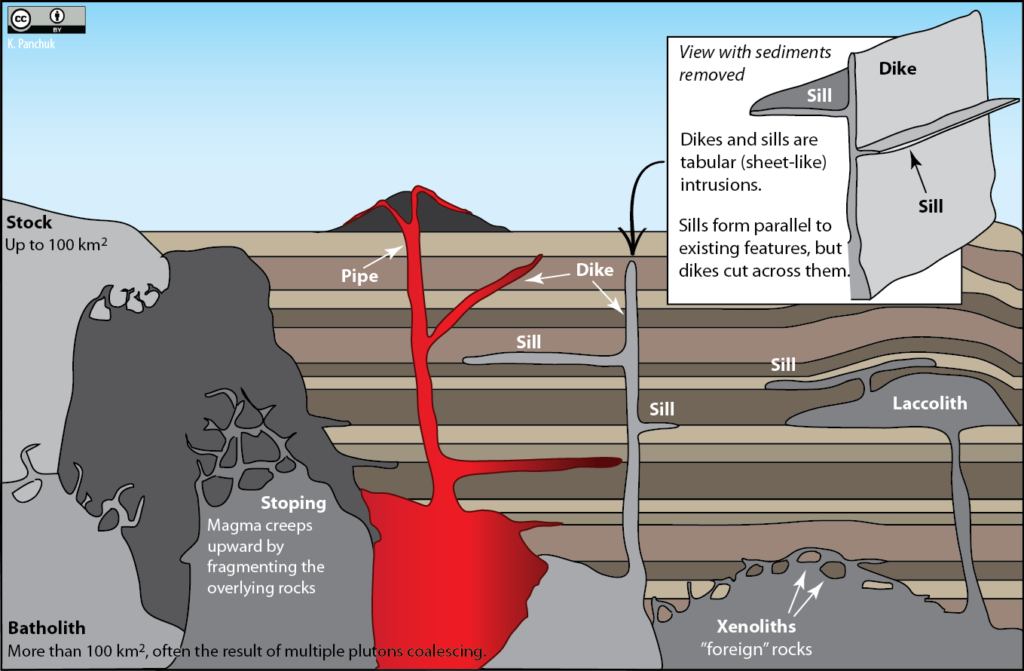
Figure 7.22: Plutons can have a variety of shapes, and be positioned in a variety of ways relative to the surrounding rocks. They are named according to these characteristics. Source: Karla Panchuk (2018) CC BY 4.0
7.4.1 Types of Plutons
7.4.1.1 Stocks and Batholiths
Large irregular-shaped plutons are called either stocks or batholiths, depending on their area. If an irregularly shaped body has an area greater than 100 km2, then it’s a batholith, otherwise it’s a stock. Note that our knowledge of the size of a body can be limited to what we see at the surface. A body with an area of less than 100 km^2 ^ exposed at the surface might in fact be much larger at depth. It might be classified as a stock initially, until someone is able to map out its true extent.
Batholiths are typically formed when a number of stocks coalesce beneath the surface to create one large body. One of the largest batholiths in the world is the Coast Range Plutonic Complex14, which extends all the way from the Vancouver region to southeastern Alaska (Figure 7.23).
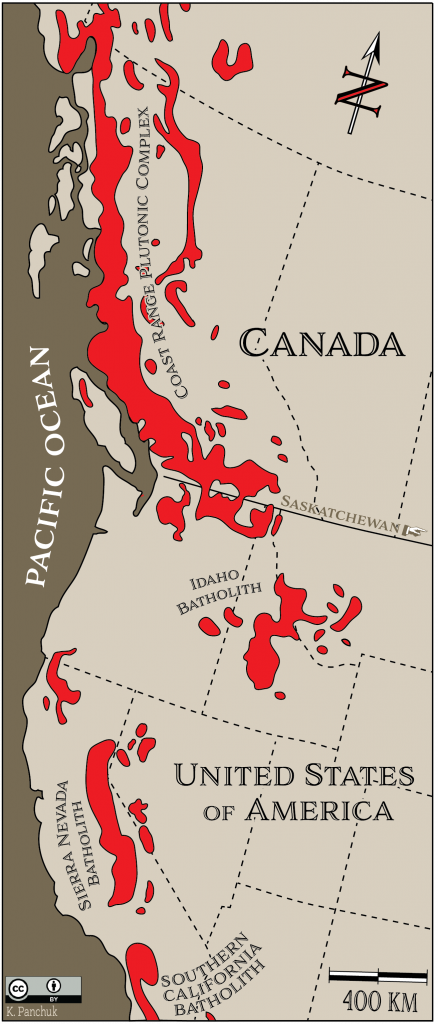
Figure 7.23: The Coast Range Plutonic Complex (also called the Coast Range Batholith) is the largest in the world. It is part of a chain of batholiths along the western coast of North America. Source: Karla Panchuk (2018) CC BY 4.0. Modified after Bally (1989).
7.4.1.2 Tabular Intrusions
Tabular (sheet-like) plutons are classified according to whether or not they are concordant with (parallel to) existing layering (e.g., sedimentary bedding or metamorphic foliation15) in the country rock. A sill is concordant with existing layering, and a dike16 is discordant. If the country rock has no bedding or foliation, then any tabular body within it is a dike. Note that the sill-versus-dike designation is not determined simply by the orientation of the feature. A dike could be horizontal and a sill could be vertical- it all depends on the orientation of features in the surrounding rocks.
A laccolith is a sill-like body that has expanded upward by deforming the overlying rock. If a sill forms, but magma pools and sags downward, it creates a lopolith.
7.4.1.3 Pipes
A pipe, as the name suggests, is a cylindrical body with a circular, elliptical, or even irregular cross-section, that serves as a conduit (or pipeline) for the movement of magma from one location to another. Pipes may feed volcanoes, but pipes can also connect plutons.
7.4.2 Chilled Margins
As discussed already, plutons can interact with the rocks into which they are intruded. Partial melting of the country rock may occur, or stoping may form xenoliths. The heat from magma can even cause causing mineralogical and textural changes in country rock. However, country rock can also affect the magma.
The most obvious effect that country rock can have on magma is a chilled margin along the edges of the pluton (Figure 7.24). The country rock is much cooler than the magma, so magma that comes into contact with the country rock cools faster than magma toward the interior of the pluton. Rapid cooling leads to smaller crystals, so the texture along the edges of the pluton is different from that of the interior of the pluton, and the colour may be darker.
_](figures/07-igneous-rocks/figure-7-24.png)
Figure 7.24: A mafic dike with chilled margins within basalt at Nanoose, B.C. The coin is 24 mm in diameter. The dike is about 25 cm across and the chilled margins are 2 cm wide. Source: Steven Earle (2015) CC BY 4.0 view source
Exercise: Pluton Problems
The diagram below is a cross-section through part of the crust showing a variety of intrusive igneous rocks. Indicate whether each of the plutons labelled a to e on the diagram below is a dike, a sill, a stock, or a batholith. (Note the trees for scale.)
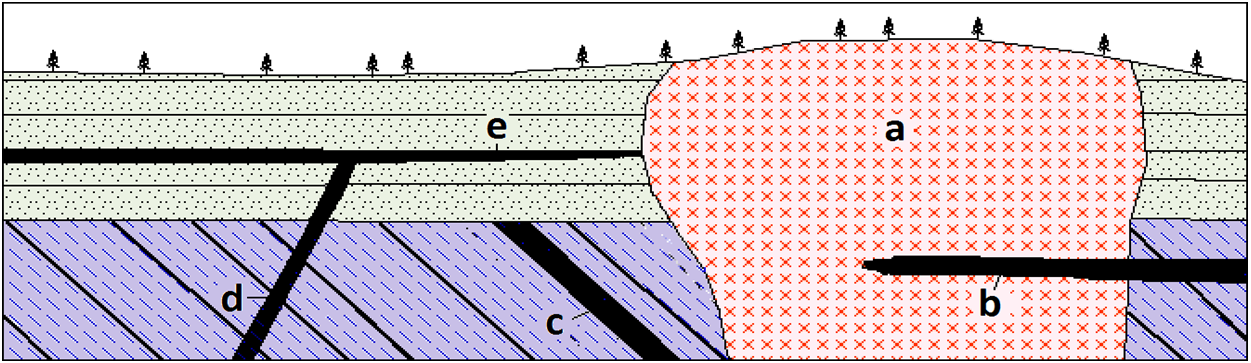](figures/07-igneous-rocks/figure-7-25.png)
Figure 7.25: A variety of igneous intrusions. Source: Steven Earle (2015) CC BY 4.0 view source
7.5 Summary
The topics covered in this chapter can be summarized as follows:
7.5.1 Magma and How It Forms
Magma is molten rock, and in most cases, it forms from partial melting of existing rock. The chemistry of magma depends on the source rock that is melting, as well as the degree of partial melting that occurs. Magma forms by decompression melting, flux-induced melting, and heat transfer. Magmas range in composition from ultramafic to felsic. Mafic rocks are rich in iron, magnesium, and calcium, and contain approximately 50% silica. Felsic rocks are richer in silica (~70%) and have lower levels of iron, magnesium, and calcium, and higher levels of sodium and potassium than mafic rocks.
7.5.2 Crystallization of Magma
As a body of magma starts to cool, the first process to take place is the polymerization of silica tetrahedra into chains. This increases the magma’s viscosity (makes it thicker) and because felsic magmas have more silica than mafic magmas, they tend to be more viscous. Bowen’s reaction series allows us to predict the order of crystallization of magma as it cools. Magma can be modified by fractional crystallization (separation of early-forming crystals), by mixing in material from the surrounding rocks by partial melting, and by mixing with magmas of differing chemistry.
7.5.3 Classification of Igneous Rocks
Igneous rocks are classified based on their mineral composition and texture. Felsic igneous rocks have less than 20% dark minerals (ferromagnesian silicates including amphibole and/or biotite) with varying amounts of quartz, both potassium and plagioclase feldspars, and sometimes muscovite. Mafic igneous rocks have more than 50% dark minerals (primarily pyroxene) plus plagioclase feldspar. Most intrusive igneous rocks are phaneritic (individual crystals are visible unmagnified). If there were two stages of cooling (slow then fast), the texture may be porphyritic (large crystals in a matrix of smaller crystals).
7.5.4 Intrusive Igneous Bodies
Magma intrudes into country rock by pushing it aside or melting through it. Intrusive igneous bodies tend to be irregular (stocks and batholiths), tabular (dikes and sills), or pipe-like. Batholiths have areas of 100 km2 or greater, while stocks are smaller. Sills are parallel to existing layering in the country rock, while dikes cut across layering. A pluton that intruded into cold rock is likely to have a chilled margin.
7.6 Chapter Review Questions
What is the significance of the term reaction in Bowen’s reaction series?
Why is it common for plagioclase crystals to be zoned from relatively calcium-rich in the middle to more sodium-rich toward the edge?
What must happen within a magma chamber for fractional crystallization to take place?
Explain the difference between aphanitic and phaneritic textures.
Name the following rocks:
- An extrusive rock with 40% Ca-rich plagioclase and 60% pyroxene
- An intrusive rock with 65% plagioclase, 25% amphibole, and 10% pyroxene
- An intrusive rock with 25% quartz, 20% potassium feldspar, 50% plagioclase feldspar, and minor amounts of biotite
What is the difference between a concordant tabular intrusion and a discordant tabular intrusion?
Why do dikes commonly have fine-grained margins?
What is the difference between a batholith and a stock?
Describe two ways in which batholiths intrude into existing rock.
Why is compositional layering a common feature of mafic plutons but not of felsic plutons?
7.7 Answers to Chapter Review Questions
As the temperature decreases minerals that formed early (e.g., olivine) may react with the remaining magma to form new minerals (e.g., pyroxene).
Calcium-rich plagioclase forms early on in the cooling process of a magma, but as the temperature drops, a more sodium-rich variety forms around the existing crystals.
Some minerals must begin to form while melt is still present. Early-forming minerals, which are typically quite dense (e.g., olivine), will sink to the bottom of the magma chamber if the magma is not too viscous, thus becoming separated from the rest of the magma. The composition of the remaining magma will be more felsic than before.
Phaneritic texture means that individual crystals can be distinguished by the naked eye. Aphanitic texture means that individual crystals cannot be distinguished without a microscope. The dividing line is somewhere between 0.1 and 1 mm, depending on the minerals.
- basalt; b) diorite; c) granite
A concordant body (a sill) is parallel to any pre-existing layering (e.g., bedding or foliation) in the country rock is. A discordant body (a dike) cuts across any pre-existing layering, or has intruded in country rock without layering (e.g., in granite).
When the hot magma intrudes into cold country rock, the margins cool quickly and small crystals form, whereas magma that is not in contact with the cool country rock will cool more slowly, and larger crystals will form. The chilled margin is the band of small crystals along the edge of the dike.
A batholith has an area of 100 km2 or greater, whereas a stock is smaller.
Batholiths (or stocks) intrude into existing rock by (a) melting through the country rock, or (b) causing the country rock to break and fall into the magma (stoping), or (c) pushing the country rock aside.
Compositional layering forms when early-crystallizing minerals sink toward the bottom of a magma chamber. This can only happen in non-viscous magma. Mafic magma is typically much less viscous than felsic magma.
7.8 References
Kushiro, I. (2007). Origins of magmas in subduction zones: a review of experimental studies. Proceedings of the Japan Academy, Series B, Physical and Biological Sciences 83(1), 1-15. Read the paper
Bally, A. W. (1989). Plate 10. Selected distribution maps, rate of accumulation maps, and lithofacies maps—Phanerozoic, North America. In A. W. Bally & A. R. Palmer (Eds.), The Geology of North America—An Overview: Volume A. Boulder: Geological Society of America.
“Mafic” combines the words __MA__gnesium and __F__errIC (containing iron).↩︎
“Felsic” combines the words __FEL__dspar and __SI__li__C__a.↩︎
The komatiites of the Song Da zone in northwestern Vietnam are 270 million years old, and those on Gorgona Island, Columbia are 89 million years old. Exactly how they formed is still a bit of a mystery. See Table 1 of arXiv:physics/0512118v2 [physics.geo-ph] for a compilation of komatiite ages with references.↩︎
From the Greek words xenos, meaning “foreigner” or “stranger,” and lithos for “stone.”↩︎
After Pluto was demoted from planet status, astronomers tried to come up with a name for objects like Pluto. For a while they considered “pluton” however geologists rightly objected that they had first claim on the word. In the end the International Astronomical Union settled on “dwarf planet” instead.↩︎
Also referred to as the Coast Range Batholith↩︎
Sedimentary bedding refers to the layers in which sedimentary rocks form. Metamorphic foliation refers to the way minerals or other elements in a rock are aligned as a result of being deformed by heat and pressure. Bedding and foliation will be discussed in more detail in later chapters.↩︎
Also spelled dyke.↩︎